Abstract
Introduction: Clonal hematopoiesis of indeterminate potential (CHIP) is defined as presence of hematologic malignancy-associated mutations, e.g. in the genes DNMT3A, TET2, ASXL1, but absence of hematologic neoplasms & is a frequent phenomenon in healthy individuals with increasing age. Some individuals with CHIP eventually develop acute myeloid leukemia (AML) & persistent clonal hematopoiesis-associated mutations (CH-mutations) in complete remission (CR) may give rise to relapse. To date in AML little is known about the biological & clinical implications of the presence of CH-mutations in CR, especially in the context of allogeneic hematopoietic stem cell transplantation (HSCT).
Patients & Methods: We analyzed peripheral blood samples of 113 AML patients (median age 63.6 years, range 31.9-75.8 years) in CR (CR1 61.9%, CR2 14.2%) or CR with incomplete peripheral recovery (CRi; 23.9%) for the presence of CH-mutations by targeted amplicon sequencing (mean amplicon coverage per sample 7205x) prior to HSCT. Genes evaluated for somatic CH-mutations based on database entries (dbSNP & COSMIC) & with a variant allele frequency (VAF) of ≥3% were DNMT3A, TET2, ASXL1, SRSF2, SF3B1, IDH1, IDH2, JAK2, PPM1D, & IKZF1 . Codon 646 ASXL1 mutations were validated using proof-reading polymerase Sanger sequencing. For 76 patients (67.3%) diagnostic bone marrow samples were available for applying a targeted amplicon panel comprising 54 recurrently mutated genes in myeloid malignancies (mean amplicon coverage per sample 8624x). At diagnosis, mutations in the genes NPM1, CEBPA, presence of FLT3 -ITD, & cytogenetics were determined using standard techniques. All patients received HSCT after non-myeloablative conditioning at our institution between 2001 & 2015. Samples were collected within 30 days before HSCT. Median follow-up for patients alive was 4.4 years after HSCT.
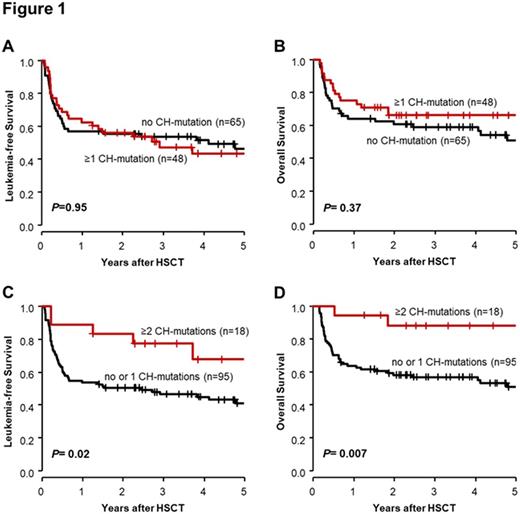
Results: We identified 70 CH-mutations present in CR/CRi in 48 AML patients (42.5%) with a mean VAF of 19.1% (58.6% of mutations with VAF>10%). The genes DNMT3A (31.4%), TET2 (28.6%) & ASXL1 (14.3%) were found mutated most frequently. We observed no associations between present CH-mutations in CR/CRi & clinical characteristics, except that patients with ≥1 CH-mutation were more often transplanted in CR2 compared to patients without CH-mutation by trend (20.8% vs 9.2%, P=0.10). The presence of ≥1 CH-mutation in CR/CRi did not impact leukemia-free survival (LFS; P=0.95, Figure 1A) or OS (P=0.37, Figure 1B), whereas patients with ≥2 CH-mutations had longer LFS (P=0.02, Figure 1C) & OS (P=0.007, Figure 1D) compared to patients with no or 1 CH-mutation. In CR/CRi DNMT3A mutations did not impact LFS (P=0.73) or OS (P=0.71), the presence of TET2 mutations did not influence LFS (P=0.25) but associated with longer OS ( P=0.06) & ASXL1 mutations associated with longer LFS (P=0.11) & OS (P=0.13) by trend. At diagnosis, we identified 83 CH-mutations in 56/76 AML patients (73.7%) of which 29 (34.9%) persisted in 19/76 patients (25.0%) in CR/CRi. Mutations in the genes JAK2 (80.0%), SRSF2 (57.1%) & DNMT3A (50.0%) were most likely to persist. We identified 9 CH-mutations (3 DNMT3A, 3 TET2, 2 ASXL1, 1 SF3B1 mutation) in CR/CRi in 8 patients (10.5%), which were not detectable at diagnosis, possibly following the expansion of subclones under chemotherapy selection pressure. Whether patients had persistent, newly detected or lost CH-mutation in CR/CRi did not impact LFS (P=0.22) or OS (P=0.83).
Conclusion: In AML patients in CR/CRi CH-mutations are frequently present (42.5%) & show high VAFs (mean 19.1%) indicating presence of clonal hematopoiesis. Compared to diagnosis 34.9% of CH-mutations persisted in CR/CRi. 10.5% of patients had CH-mutations only detectable in CR/CRi prior to HSCT. While we did not observe a negative prognostic impact of presence of ≥1 CH-mutation in CR/CRi, the presence of distinct mutations (TET2, ASXL1) may positively influence outcome. Patients with ≥2 CH-mutations had longer LFS & OS. This finding may be explained by an increased immunogenic potential with higher number of CH-mutations leading to potent graft vs leukemia effects in the HSCT context.
Lange: Novartis: Honoraria, Research Funding. Franke: Takeda: Consultancy; Pfizer: Honoraria; Novartis: Honoraria, Research Funding. Schwind: Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal